The group of Dr. Bin Zhao published a research article on Science Advances online on June 22, 2022 with the title “Liver cancer heterogeneity modeled by in situ genome editing of hepatocytes”. Dr. Bin Zhao and Dr. Tingbo Liang are co-corresponding authors and graduate students Mei Tang, Yang Zhao and Jianhui Zhao are co-first authors.
Primary liver cancer (PLC) is the fourth leading cause of cancer-related mortality worldwide, and treatments are very limited. Hepatocellular carcinoma (HCC) accounts for 75%-85% of PLC, and intrahepatic cholangiocarcinoma (ICC) makes up 10%-15%, besides other rare types. 30-40% of HCC patients may be eligible for surgical resection or transplantation. But for advanced HCCs, only a few multi-kinase inhibitors providing very limited survival benefits are available. ICC is more difficult to diagnose and to treat compared with HCC, and has worse prognosis. While much hope has been placed in the identification of novel targets through molecular profiling, it was obscured by the heterogeneity in the cause, genetics, and phenotypes of PLC. Currently it is of great significance to develop pre-clinical models that well reflecting heterogeneity of liver cancer to study the mechanism of heterogeneity and preclinical testing.
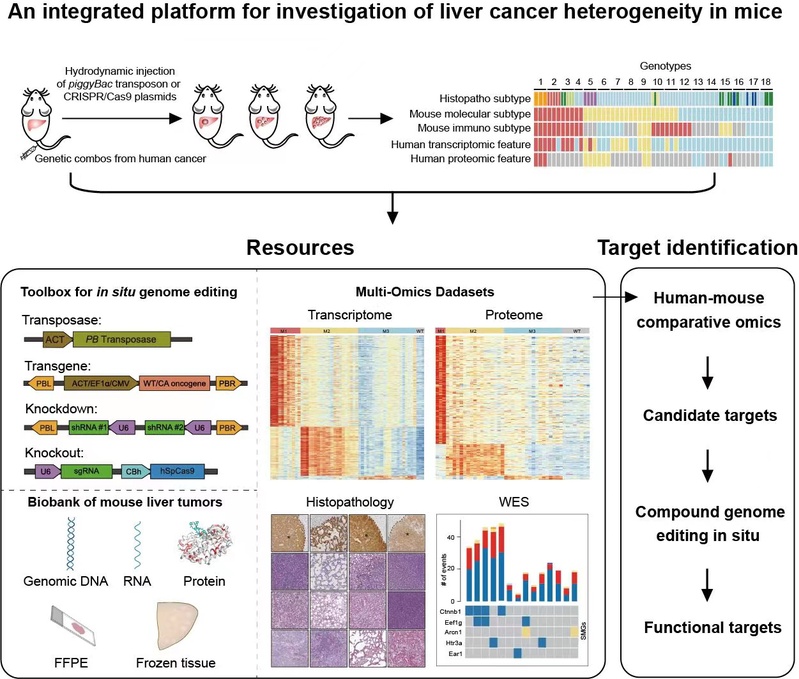
In order to induce liver tumors representing heterogenous phenotypes as a result of diverse genetic drivers, the authors first consulted the mutational landscape of HCC. Twenty-two potential driver genes were selected by mutational frequency from 7 human HCC cohorts with diverse etiological factors and ethnic origins. Next the authors generated a plasmid toolbox for expression of transgenes in piggyBac (PB) transposons, as well as sgRNAs and Cas9 for in situ genome editing of mouse hepatocytes, which were delivered into mouse hepatocytes through hydrodynamic injection. Primary mouse liver tumors in 25 genotypes were produced.
For further study whether the model reflects the heterogeneity of liver cancer, the authors conducted a comprehensive analysis of the mouse live tumors. Histopathological analysis showed that these mouse tumors represent major histopathological types of human PLCs and the tumor grade and specific pathological features in human PLC were also simulated in the models. Whole exon sequencing had revealed different mutational patterns in mouse tumors. Hotspot mutations of Ctnnb1 in exon 3 were frequently found in F/C and PI3K+F/C tumors. Various mutant alleles were commonly found in a single tumor and heterogenous nuclear accumulation of Ctnnb1 was observed. Further transcriptomic and proteomic analysis identified three human-matched molecular subtypes of mouse tumors named M1-M3. Importantly, phenotypical characterization identified subtype- or genotype-specific alterations in immune microenvironment, metabolic reprogramming, cell proliferation, and expression of drug targets. M1 mouse liver tumors were similar to those of respective human subtypes with features of hepatocyte dedifferentiation and ICC-like properties, remodeling of the cytoskeleton and related fibrosis, and heavy infiltration of immune cells. The M2 subtype was featured by highly proliferative hepatic stem cell-like HCC. The M3 subtype was featured by retention of liver function and liver metabolism. Furthermore, single cell analysis and expression tracing revealed spatial and temporal dynamics in expression of pyruvate kinase M2 (Pkm2). Tumor-specific knockdown of Pkm2 by multiplexed genome editing reversed Warburg effect and suppressed tumorigenesis in a genotype-specific manner.
In summary, they report an integrated resource comprising an expandable plasmid toolbox for in situ genome editing of mouse hepatocytes, a biobank of mouse liver tumors in different genotypes, as well as multi-omics datasets revealing human-matched subtypes of mouse liver tumors. This resource would be valuable for mechanistic study of heterogenous abnormalities in liver cancer, and for evaluation of novel therapies in a preclinical setting, which may thus fill the gap in translating molecular subtyping to clinics.
Link:https://www.science.org/doi/10.1126/sciadv.abn5683